Intersectoral action: Public–private collaboration to improve equitable access to vasopressors for septic shock
Authors: Leong Trudy1, Mpofu Rephaim2, Kredo Tamara1, 3
1. Health Systems Research Unit, South African Medical Research Council, Cape Town, South Africa
2. Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa
3. National Essential Medicines List Committee, National Department of Health, Pretoria, South Africa
Introduction
Health inequity in South Africa
- South Africa's health system is emblematic of the country's deep socio-economic inequalities, caused by a complex interplay of historical, economic, and social factors—also exacerbated by systemic challenges within the health sector and the growing quadruple disease burden.
- The country’s GINI coefficient is one of the highest in the world, reflecting stark disparities in wealth distribution, mirrored in South Africa's two-tiered public–private health system (@TIME’sInequalityInSA, @WorldBankInequality).
- The public sector services approximately 84% of the population and is underfunded, while private medical insurance covers 16%, providing access to well-equipped facilities, but at a higher cost.
- This inequity contributes to unequal health outcomes, hinders social mobility, and can fuel social tension.
Access to essential medicines
- Access to affordable health services of appropriate quality, including essential medicines, has an important influence on health inequities.
- For the public sector, the ministerially appointed National Essential Medicines List Committee (NEMLC) decides which essential medicines are accessed through the South African Essential Medicines List (EML).
- For the private-sector, the Council for Medical Schemes, provisioned by Act 131 of 1998, has determined a set of Prescribed Minimum Benefits (PMBs) that all private insurers must provide. These PMBs are often guided by decisions made by the NEMLC.
- Public-sector medicines are procured through tenders at prices generally lower than the legislative single exit price guiding access to private-sector medicines.

Background
National Health Insurance Act
- In May 2024, South Africa’s National Health Insurance (NHI) Act was signed into law to support Universal Healthcare Coverage—a health funding system that aims to provide access to affordable, high-quality health care for all South Africans, irrespective of their socio-economic status.
- However, collaboration between the public (including academia) and private sectors is crucial. Intersectoral action in health is the coordinated efforts of different sectors to address and improve public health outcomes.
- We will focus on public–private collaboration to assess medicines for inclusion in the EML. The assessment process is called Health Technology Assessment (HTA)—a multidisciplinary evidence-informed process evaluating the social, economic, organizational, and ethical issues related to the use of health technologies, including medicines.
- Essential medicines and the HTA process are mentioned in the NHI Act.
Why should public and private sectors work together?
- Bringing together diverse perspectives, expertise, and resources from public and private sectors will lead to more comprehensive, credible, and contextually relevant medicine assessments that are more widely accepted and implemented, ultimately improving the quality of evidence-informed decisions. This will ensure the effective and equitable use of medicines (@WHOPublicPrivatePartnership).
- Ensuring good governance with transparency, efficiency, and accountability, intersectoral collaboration ensures that HTA serves as a powerful tool to optimize South Africa’s health care outcomes and resource allocation (@EthicalGovernance).
Working together: The case of vasopressors for septic shock
- The Primary Healthcare/Adult Hospital sub-committee of NEMLC, academia, and clinicians from the Critical Care Society of Southern Africa worked together to produce Standard Treatment Guidelines for critical care.
- One of the areas of concern was the choice of vasopressor to manage septic shock—where widespread infection leads to dangerously low blood pressure and organ dysfunction. Vasopressors (such as norepinephrine, epinephrine, dopamine, and vasopressin) help maintain adequate blood flow to vital organs.
The problem
Vasopressor debate in the Surviving Sepsis Guidelines
- The Surviving Sepsis Campaign (SSC) guidelines are globally recognized for managing sepsis and septic shock but have faced controversies and criticisms, especially regarding their applicability to low- and middle-income countries (LMICs).
- An area of debate is the use of vasopressors for treating septic shock. The SSC guidelines recommend norepinephrine as the first-line vasopressor, with epinephrine as the second-line option. However, some argue that norepinephrine and epinephrine are both effective, with unclear evidence of superiority.
- The SCC guidelines raise concerns about epinephrine’s potential to increase lactate levels (complicating the clinical assessment of sepsis severity) and heart rates. However, some argue that there is limited evidence that epinephrine is associated with poorer outcomes based on elevated lactate. Rather, epinephrine may have off-target pharmacological effects that increase lactate production, and hence, lactate may not be a reliable predictor or marker for improved organ perfusion in this setting.
- Currently, the South African public sector guidelines recommend epinephrine for septic shock, accessible through a public sector tender. In contrast, the private sector can access epinephrine and norepinephrine, but at higher single exit prices.
Teamwork
A team of experts therefore set out to review the evidence comparing the effectiveness and safety of epinephrine to norepinephrine in the initial management of septic shock in adults (@UHCtogether).
Reviewing the evidence
Systematic reviews and meta-analyses are highly regarded for providing high-quality, evidence-based insights into research questions.
- A systematic review is a structured approach reviewing published literature on a specific topic. The methodological process is transparent and reproducible, aiming to minimize bias using clear criteria for study selection and appraisal (see Figure 1).
A meta-analysis is a statistical method combining quantitative data from multiple studies to improve statistical power, provide a more precise estimate of the measured outcome, and explore variability among the selected studies.
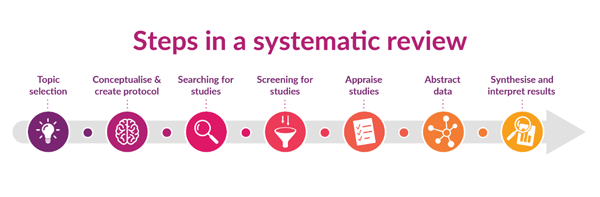
Figure 1: Creative Commons BY-SA - Illustration created by Karolinska Institute University Library.
The search for answers
Research methodology
- We defined a clear research question with set criteria for studies to be reviewed.
- To minimize duplication of efforts and improve efficiency, we first searched for other good-quality clinical practice guidelines and appropriate HTAs that matched our research question. We found that international guidelines largely align with the SSC Guidelines, recommending norepinephrine as the initial vasopressor of choice.
- As no other good-quality guidelines or HTAs could be found, we conducted a systematic review and meta-analysis of randomized controlled clinical trial data published in peer-reviewed scientific journals.
- We also reviewed previous SSC Guidelines (2001, 2004, 2008, 2012, and 2016) to assess the rationale and evidence for selecting norepinephrine, preferred over epinephrine, and did not identify any additional studies.
Research findings
- A review of the evidence showed little difference between norepinephrine and epinephrine. Limited available randomized controlled trial evidence suggests that the mortality risk and time required to stabilize blood pressure were similar for both agents.
- Furthermore, the risks of abnormal or increased heart rate and increased lactate levels in the blood (which usually indicates that the body’s cells are not getting enough oxygen) appeared to be similar for both agents, although the evidence is very limited with very low certainty.
- The increase in lactate concentrations appears to be transient and may not be clinically significant.
- The current 2021 SSC Guidelines recommend norepinephrine as the vasopressor of choice but there is very limited evidence for this. Our systematic review and meta-analyses suggest that either norepinephrine or epinephrine can be used as the primary vasopressor of choice for the initial management of septic shock in adults.
- The choice should further be informed by the affordability and cost of the agent, the feasibility of implementing the strategy, and the accessibility of the agent. A direct comparison of per-milligram drug prices showed a 7- to -fold increase in cost to use norepinephrine at clinically effective doses compared to epinephrine.
Research conclusion
- This review provides reassurance of the current public sector guideline, recommending epinephrine as the initial vasopressor treatment option for septic shock.
- Valuable insights were gained for public-sector guideline development in South Africa.
- However, further research is needed to determine when norepinephrine and/or other vasopressors may be necessary, especially where epinephrine is inappropriate or insufficient.
Potential global impact
Adapt rather than adopt global guidelines
- The SCC Guidelines have improved awareness and management of sepsis, but there has been much debate about the recommendations and the underpinning research. Critics argue that these guidelines should not be merely adopted, but appraised, applied with clinical judgment, adapted to local contextual evidence with consideration of resources of the local health care system, and flexibly applied to individual patient care.
- In the evidence ecosystem, evidence of efficacy and safety is usually generated globally from multi-country randomized controlled trials. However, contextual considerations are required to ensure effective implementation, sustainability, and ultimately, improved health outcomes for the respective population.
Lessons learned
Intersectoral collaboration between different socio-economic sectors to promote equity
- To ensure global applicability, SCC guideline development should involve collaboration between high-income countries and LMICs.
- Consideration should be given to social determinants of health and the socio-economic impact of various sectors for HTA processes to improve universal access to affordable health care of appropriate quality for septic shock.
- Intersectoral action can lead to more comprehensive, practical, and sustainable public health policies and guidelines for diverse populations. Such collaboration/action is likely to promote the quality of care for patients with sepsis and improve patient outcomes globally.
- However, the process could be strengthened by involving indigenous public representative groups to assess population needs and cultural values.
Next steps
The first step toward meaningful collaboration in the South African health care sector is to break down the barriers between the public and private sectors. This will help ensure that decisions are made with a full understanding of the country's significant health care challenges, given the country's stark variance in equity and quadruple disease burden. Additionally, it is important to involve sectors beyond health care in addressing the social determinants of health.

References
Becker, J. U., Theodosis, C., Jacob, S. T., Wira, C. R., & Groce, N. E. (2009). Surviving sepsis in low-income and middle-income countries: new directions for care and research. The Lancet. Infectious diseases, 9(9), 577–582. https://doi.org/10.1016/S1473-3099(09)70135-5
Buse, K., & Waxman, A. (2001). Public-private health partnerships: a strategy for WHO. Bulletin of the World Health Organization, 79(8), 748–754.
Council for Medical Schemes. (2024). What are Prescribed Minimum Benefits? https://www.medicalschemes.co.za/resources/pmb/
de Villiers K. (2021). Bridging the health inequality gap: an examination of South Africa's social innovation in health landscape. Infectious Diseases of Poverty, 10(1), 19. https://doi.org/10.1186/s40249-021-00804-9
van, L., Rhodes, A., Alhazzani, W., Antonelli, M., Coopersmith, C. M., French, C., Machado, F. R., Mcintyre, L., Ostermann, M., Prescott, H. C., Schorr, C., Simpson, S., Wiersinga, W. J., Alshamsi, F., Angus, D. C., Arabi, Y., Azevedo, L., Beale, R., Beilman, G., Belley-Cote, E., … Levy, M. (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine, 47(11), 1181–1247. https://doi.org/10.1007/s00134-021-06506-y
Garcia-Alvarez, M., Marik, P., & Bellomo, R. (2014). Sepsis-associated hyperlactatemia. Critical care (London, England), 18(5), 503. https://doi.org/10.1186/s13054-014-0503-3
Jones, A. E., Shapiro, N. I., Trzeciak, S., Arnold, R. C., Claremont, H. A., Kline, J. A., & Emergency Medicine Shock Research Network (EMShockNet) Investigators (2010). Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA, 303(8), 739–746. https://doi.org/10.1001/jama.2010.158
Mantsiou, C., Liakos, A., Mainou, M., Papanas, N., Tsapas, A., & Bekiari, E. (2023). A Simple Guide to Systematic Reviews and Meta-Analyses. The International Journal of Lower Extremity Wounds, 15347346231169842. Advance online publication. https://doi.org/10.1177/15347346231169842
McMaster Health Forum on behalf McMaster University: The Evidence Commission report. 4.4 Interplay of local and global evidence. https://www.mcmasterforum.org/docs/default-source/evidence-commission/sections/4.4-interplay-of-local-and-global-evidence.pdf?sfvrsn=615657ea_17
Moja, L., & Huttner, B. (2023). Selection and use of essential medicines. In Global Health Essentials 2023 Sep 8 (pp. 321-325). Cham: Springer International Publishing.
Morillon M. (2024). Understanding Health Technology Assessment (HTA): Process, steps, and real-world examples. https://pro.carenity.com/library/hta-definition/
National Department of Health. Health Technology Assessment Methods Guide, 2022-2027. https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-11/HTA%20Methods%20Guide_FINAL_Sep%202023.pdf
National Essential Medicines List Committee. (2022). Terms of reference. https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-02/NEMLC%20TOR_Final_20Oct2022.pdf
Ndumbe-Eyoh, S., & Moffatt, H. (2013). Intersectoral action for health equity: a rapid systematic review. BMC Public Health, 13, 1056. https://doi.org/10.1186/1471-2458-13-1056
Omar, S., Burchard, A. T., Lundgren, A. C., Mathivha, L. R., & Dulhunty, J. M. (2011). The relationship between blood lactate and survival following the use of adrenaline in the treatment of septic shock. Anaesthesia and Intensive Care, 39(3), 449–455. https://doi.org/10.1177/0310057X1103900316
Pomeranz K. (2019). The story behind TIME’s Cover on inequality in South Africa. Time. https://time.com/5581483/time-cover-south-africa/
Universal Health Coverage. (2023). Private sector commitments to universal health coverage: UHC 2030 private sector constituency 2023 statement. https://www.uhc2030.org/fileadmin/uploads/UHC2030_Private_Sector_Commitments_Statement_April2023.pdf
Sartini, C., Landoni, G., Belletti, A., Kotani, Y., Maimeri, N., Umbrello, M., Yavorovskiy, A., & Jabaudon, M. (2024). Beyond the Surviving Sepsis Campaign Guidelines: a systematic review of interventions affecting mortality in sepsis. Panminerva medica, 66(1), 55–62. https://doi.org/10.23736/S0031-0808.23.04986-8
Schünemann, H. J., Wiercioch, W., Brozek, J., Etxeandia-Ikobaltzeta, I., Mustafa, R. A., Manja, V., Brignardello-Petersen, R., Neumann, I., Falavigna, M., Alhazzani, W., Santesso, N., Zhang, Y., Meerpohl, J. J., Morgan, R. L., Rochwerg, B., Darzi, A., Rojas, M. X., Carrasco-Labra, A., Adi, Y., AlRayees, Z., … Akl, E. A. (2017). GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. Journal of Clinical Epidemiology, 81, 101–110. https://doi.org/10.1016/j.jclinepi.2016.09.009 Standard Treatment Guidelines (Hospital Level) for Adult Critical Care, 2024 and supporting evidence reviews. https://knowledgehub.health.gov.za/system/files/elibdownloads/2024-04/Adult%20Hospital%20Chapter%2023%20-Critical%20Care_with%20supporting%20NEMLC%20report_2020-3%20review.pdf
Thaver, T., Padayachee, N., & Bangalee, V. (2021). A comparison study between public and private healthcare sector medicine prices in South Africa. Journal of Pharmaceutical Health Services Research, 12(2), 194-205. https://doi: 10.1093/jphsr/rmaa016
The National Health Insurance Act 20 of 2023. https://www.gov.za/documents/acts/national-health-insurance-act-20-2023-english-afrikaans-16-may-2024
Tomas, M. (2021). The basics of good governance. https://www.good-governance.org.uk/publications/insights/the-basics-of-good-governance
Trowbridge, J., Tan, J. Y., Hussain, S., Osman, A. E. B., & Di Ruggiero, E. (2022). Examining Intersectoral Action as an Approach to Implementing Multistakeholder Collaborations to Achieve the Sustainable Development Goals. International journal of public health, 67, 1604351. https://doi.org/10.3389/ijph.2022.1604351
Wilkinson, M., Gray, A. L., Wiseman, R., Kredo, T., Cohen, K., Miot, J., Blecher, M., Ruff, P., Johnson, Y., Poluta, M., McGee, S., Leong, T. D., Brand, M., Suleman, F., Maramba, E., Blockman, M., Jugathpal, J., Cleary, S., Nematswerani, N., Moodliar, S., … Wilkinson, T. (2022). Health Technology Assessment in Support of National Health Insurance in South Africa. International journal of technology assessment in health care, 38(1), e44. https://doi.org/10.1017/S0266462322000265
World Bank. (2016). Poverty and shared prosperity 2016: Taking on inequality. Washington, DC: World Bank. doi:10.1596/978-1-4648-0958-3.
World Health Organization. (2024). Essential medicines. https://www.who.int/westernpacific/health-topics/essential-medicines#tab=tab_1
World Health Organization. (2024). Social determinants of health. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
To link to this article - DOI: https://doi.org/10.70253/KSLG9525
Links to additional resources
National Department of Health. Essential Drugs Programme. https://www.health.gov.za/nhi-hpp-edp/
Critical Care Society of South Africa: https://criticalcare.org.za/
Health Systems Research Unit, South African Medical Research Council: https://www.samrc.ac.za/research/intramural-research-units/HealthSystems
Co-publication declaration
The NEMLC-approved evidence review (norepinephrine compared to epinephrine for adult septic shock) is published on the National Department of Health Knowledge Hub platform, and is available at: Knowledge Hub link
Acknowledgments
Review team: Leong TD, Dadan S, Griesel R, Mpofu R
National Department of Health, Essential Drugs Programme, Pretoria, South Africa
Critical Care Society of South Africa
National Essential Medicines List Committee (NEMLC), Pretoria, South Africa
PHC/ Adult Hospital Level Expert Review Committee, Pretoria, South Africa
Editorial support: Michelle Galloway (Health Systems Research Unit, South African Medical Research Council)
Disclaimer
The views expressed in this World EBHC Day Blog, as well as any errors or omissions, are the sole responsibility of the author and do not represent the views of the World EBHC Day Steering Committee, Official Partners or Sponsors; nor does it imply endorsement by the aforementioned parties.
